Smart-CKD: Elevating Chronic Kidney Disease Management with a Non-invasive Diagnostic Tool Integrating Demographic Data with Ultrasound Features
Other Articles

Harnessing Ultrasound and Machine Learning to Assess Renal Fibrosis and Improve Patient Outcomes
Study conducted by Prof. Michael Tin-cheung YINGand his research team

Chronic Kidney Disease (CKD) is a significant global health challenge, with its prevalence steadily increasing and its impact on global mortality becoming more pronounced. According to the World Health Organization, CKD accounts for 1.5% of deaths worldwide. In Hong Kong alone, over 800,000 individuals suffer from CKD. CKD is characterised by the gradual loss of kidney function, ultimately leading to end-stage renal disease, which necessitates dialysis or kidney transplantation. As of the end of 2023, more than 2,400 patients in Hong Kong remained on the kidney transplant waiting list.
Renal fibrosis is a key driver of CKD progression, characterised by excessive extracellular matrix deposition and loss of functional kidney tissue. Accurate assessment of renal fibrosis is crucial for effective CKD management; however, traditional methods such as renal biopsy, despite being the gold standard, are invasive and carry risks that include serious bleeding. Repeated renal biopsies for follow-up monitoring are impractical. Ultrasound (US) provides a non-invasive, cost-effective alternative but its conventional use has limitations in providing comprehensive diagnostic information.
Prof. Tin-cheung YING, Associate Head and Professor of the Department of Health Technology and Informatics at The Hong Kong Polytechnic University, recognised the urgent need for a reliable, non-invasive diagnostic tool and led the development of Smart-CKD (S-CKD)—a novel computer-aided system that integrates demographic data with US features to assess fibrosis severity in CKD patients.
Published in Academic Radiology, Prof. Ying and his research team introduced the S-CKD tool as a significant advancement in CKD management, leveraging machine learning algorithms alongside conventional US technology. The team employed the Least Absolute Shrinkage and Selection Operator (LASSO) regression algorithm, a highly effective method for handling small datasets with numerous variables, to identify US imaging features with the highest diagnostic value. This approach ensured that only the most relevant variables—age, renal length, and end-diastolic velocity of the interlobar renal artery—were retained and integrated into a multivariate logistic regression model. The S-CKD model was validated using receiver operating characteristic curves, demonstrating favourable diagnostic performance with an area under the curve of 0.84 in the training cohort and 0.81 in the validation cohort.
The tool's performance was further validated through calibration curves and the Hosmer-Lemeshow test, confirming close agreement between predicted and observed outcomes. Additionally, decision curve analysis highlighted the clinical utility of S-CKD, showing a higher net benefit compared to treat-all or treat-none approaches across various risk thresholds. To enhance accessibility, the S-CKD tool was deployed as both an online web-based application and an offline document-based adjunct, ensuring usability across diverse clinical settings, including in areas with limited internet access.
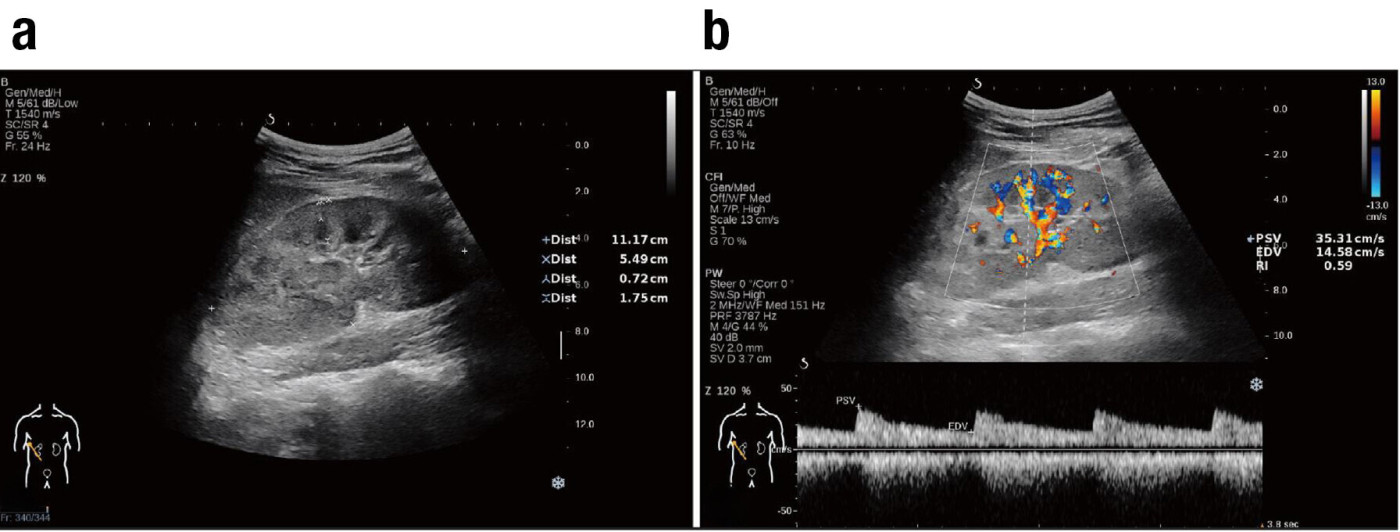
Figure 1. Ultrasound assessment of the kidney
a) Measurement of renal length, width, parenchyma thickness, and cortical thickness using B-mode ultrasound
b) Assessment of PSV, EDV, and RI using colour Doppler ultrasound
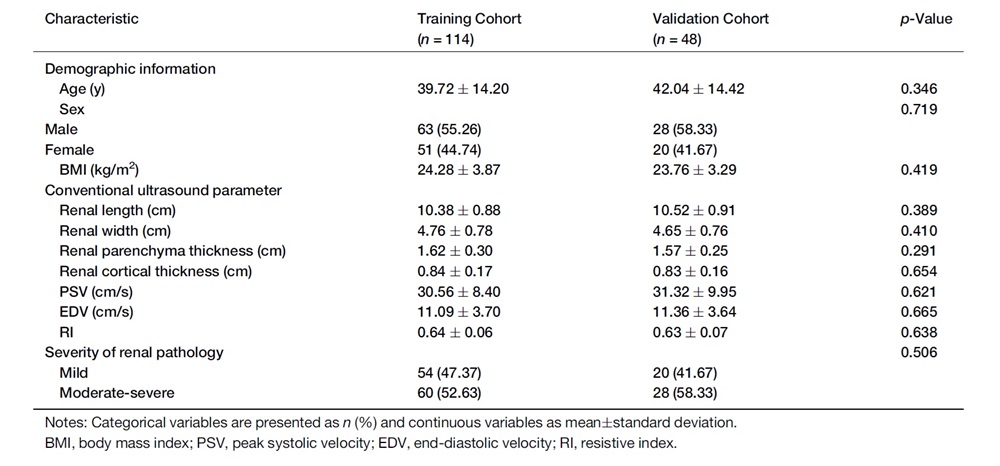
Table 1. Baseline characteristics of CKD patients in the training and validation cohorts
The development of the S-CKD tool involved a rigorous process of feature selection and model construction. A total of 162 CKD patients undergoing renal biopsy and US examinations was enrolled on the study and randomly assigned to either the training group (n = 114) or validation group (n = 48). US imaging was performed on the kidney to obtain renal size parameters (Figure 1a), followed by measurement of blood flow indices in the interlobar renal artery using Color Doppler US (Figure 1b). Patient characteristics for both groups are shown in Table 1. The LASSO regression algorithm was employed to screen out redundant variables from demographic and US characteristics, retaining only those with strong diagnostic value. This process identified age, renal length, and EDV as the most significant diagnostic variables for moderate-severe renal fibrosis (Table 2).
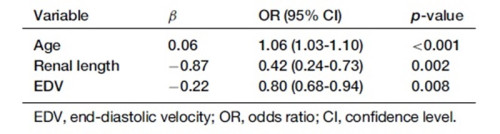
Table 2. Risk factors for renal fibrosis severity
Once identified, the key features were used to construct the S-CKD tool via a multivariate logistic regression model. The risk of developing moderate-severe renal fibrosis was found to increase with advancing age, a reduction in renal length, or EDV. Specifically, each additional year of age led to a 6% increase in risk, while the risk increased by 58% and 20%, respectively, for every one-unit reduction in renal length and EDV. These findings were incorporated into the S-CKD risk calculator, enabling clinicians to estimate fibrosis severity in CKD patients. The S-CKD tool was deployed as an online, web-based auxiliary diagnostic device, allowing clinicians to evaluate the probability of moderate-severe renal fibrosis in CKD patients (Figure 2a). An offline document-based adjunct was also developed to ensure accessibility in areas with poor internet access (Figure 2b).
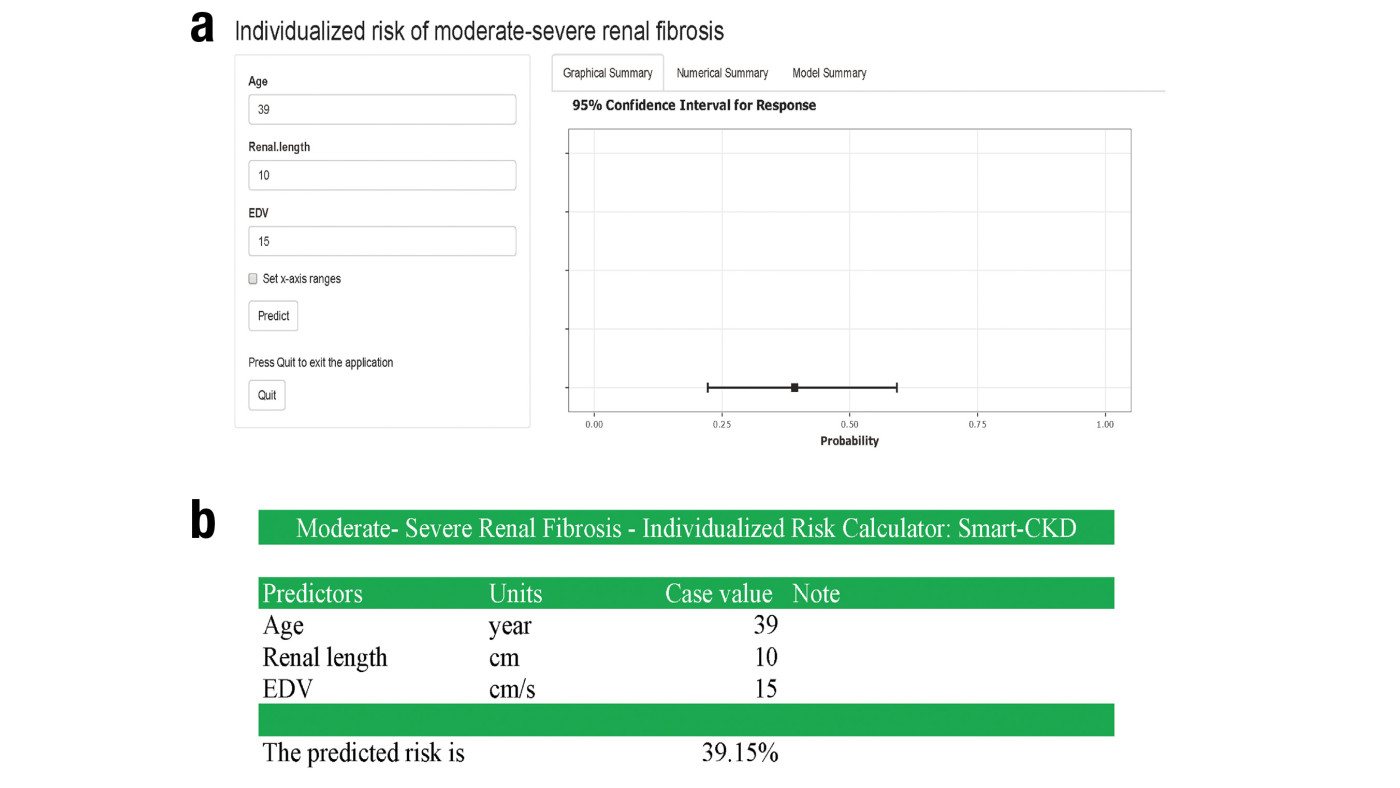
Figure 2. The S-CKD diagnosis tool
a) The online, web-based version of the tool
b) The offline, document-based version of the tool
The performance of S-CKD was validated, demonstrating satisfactory diagnostic performance, with decision curve analysis further confirming its superior clinical net benefit.
S-CKD marks a transformative step in CKD diagnosis, offering a practical, non-invasive, and accessible alternative to traditional diagnostic methods. By integrating demographic and ultrasound data, it provides a robust framework for risk assessment, guiding treatment decisions, and improving patient outcomes. As CKD continues to pose a significant health burden worldwide, innovations like S-CKD play a crucial role in advancing diagnostic capabilities and enhancing clinical care. Future research should focus on validating S-CKD in larger, multi-centre cohorts and exploring its integration into clinical workflows to maximise its impact on patient care. The study data are available on reasonable request from the corresponding authors. The data are not publicly available due to ethical privacy restrictions. The work was supported by the Natural Science Foundation of Guangdong Province (2018A0303130070) and National Natural Science Foundation of China (82072038).
Prof. Ying has been recognised by Stanford University as one of the top 2% most-cited scientists worldwide (single-year) in the field of nuclear medicine & medical imaging for two consecutive years, from 2023 to 2024. Prof. Ying is currently the Associate Head of the Department of Health Technology and Informatics of The Hong Kong Polytechnic University.
| References |
|---|
[1] Chen, Z., Chen, J., Ying., T. C., Chen, H., Wu, C., Chen, X., Huang, Y. & Su, Z. (2023). Development and Deployment of a Novel Diagnostic Tool Based on Conventional Ultrasound for Fibrosis Assessment in Chronic Kidney Disease. Academic Radiology, Volume 30, Supplement 1, September 2023, Pages S295-S304. https://doi.org/10.1016/j.acra.2023.02.018
 | Prof. Michael Tin-cheung YING |




