Hope for Bone Repair and Regeneration: Enhancing Osteogenesis with 3D-Printed Triply Periodic Minimal Surface Scaffolds
Other Articles

A Novel Approach in Bone Tissue Engineering
Study conducted by Prof. Xin ZHAOand her research team

Bone tissue engineering has long sought innovative solutions to improve the efficacy of bone regeneration. Conventional scaffolds often face limitations in stress concentration and biocompatibility such as supporting the attachment, proliferation, osteogenic differentiation, and angiogenic paracrine function of human mesenchymal stem cells. These factors can hinder scaffold effectiveness in clinical applications.
In a research article published in Proceedings of the National Academy of Sciences (PNAS), Prof. Xin ZHAO, Professor of the Department of Applied Biology and Chemical Technology at The Hong Kong Polytechnic University, and her research team explored the use of triply periodic minimal surface (TPMS) scaffolds, designed with varying Gaussian curvatures, to address these challenges [1]. By leveraging advanced 3D printing techniques and the unique geometric properties of TPMS, the team aims to enhance osteogenic differentiation and angiogenesis, offering a promising pathway for bone repair and regeneration.
The significance of this research lies in its potential to revolutionise bone grafting techniques. Inspired by the natural structure of leaf photosynthesis, coral mineralisation and trabecular bone grow, TPMS scaffolds, with their intricate hyperboloidal topography, mimic the natural extracellular matrix more closely than conventional designs (Figure 1). This biomimetic approach not only improves mechanical properties but also influences cellular behaviour, directing stem cell fate towards osteogenesis and angiogenesis. The ability to tailor scaffold geometry to optimise these outcomes represents a significant advancement in personalised medicine and regenerative therapies.
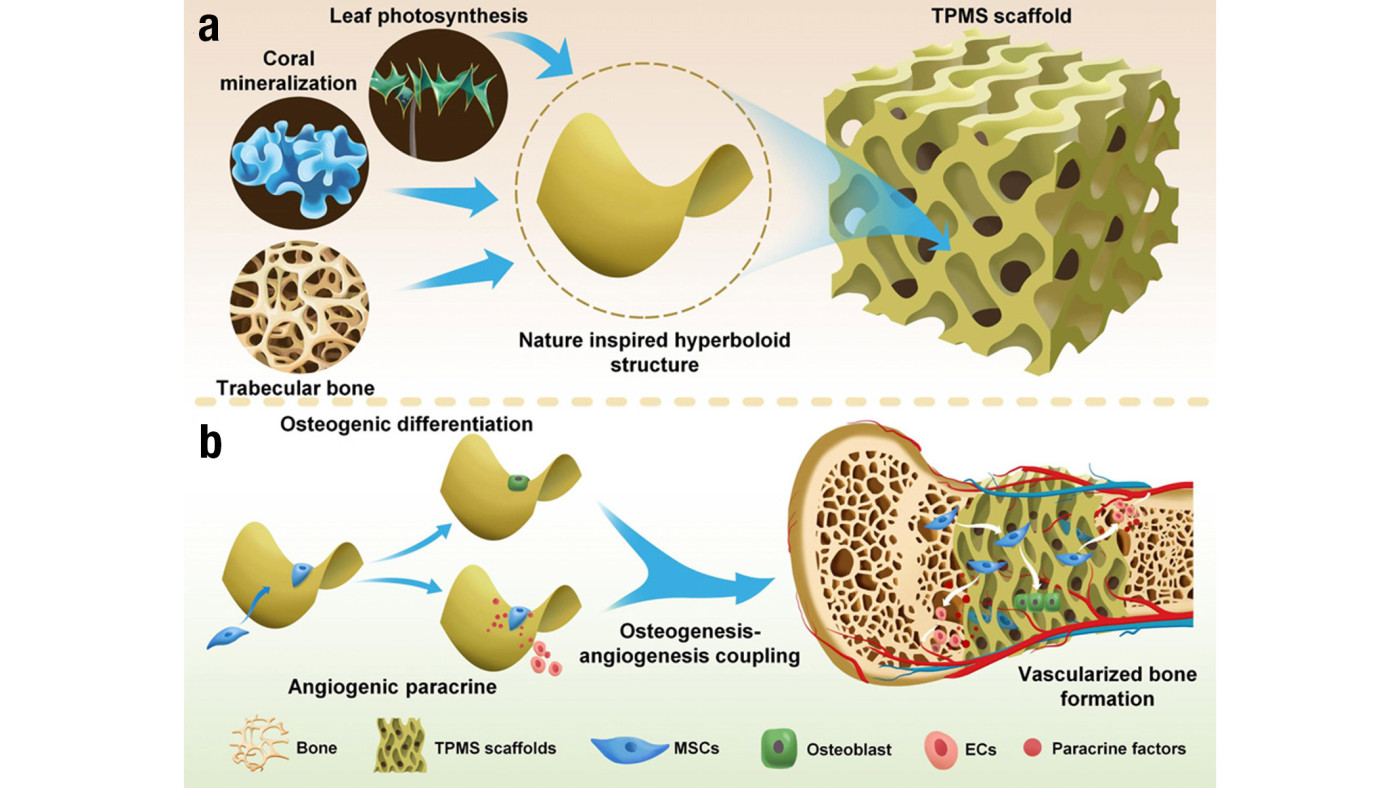
Figure 1. Schematics show the design and application of the 3D TPMS scaffolds.
a) The natural microstructure of leaf photosynthesis, coral mineralisation and trabecular bone inspired the hyperboloid design of TPMS scaffolds.
b) The behaviours and functions of mesenchymal stem cells (MSCs) in terms of osteogenic differentiation and angiogenic paracrine for osteogenesis-angiogenesis coupling and vascularised bone regeneration can be regulated on TPMS scaffolds with hyperboloidal surfaces.
Prof. Zhao and the team designed TPMS scaffolds with Gaussian curvatures of –2, –4 and –6 mm-2, utilising stereolithography-based 3D printing and sintering to fabricate structures composed of body-inherent β-tricalcium phosphate (β-TCP) (Figure 2a&b). The factors were chosen as they were in range of natural trabecular bone. Through meticulous optimisation of printing and sintering parameters, the team achieved precise control over scaffold architecture, ensuring high accuracy and reproducibility. Representative scanning electron microscope images of the fabricated scaffolds showed smooth and dense surfaces with no visible defects and high resolution of the printing process, with an accurate recapitulation of the hyperboloidal topology (Figure 2c). The spatially internal structure of the scaffolds was evaluated by high-resolution micro-computed tomography analysis (Figure 2d&e). The scaffolds maintained a consistent porosity of approximately 60%, facilitating the interconnected pore networks essential for nutrient flow and cell migration.
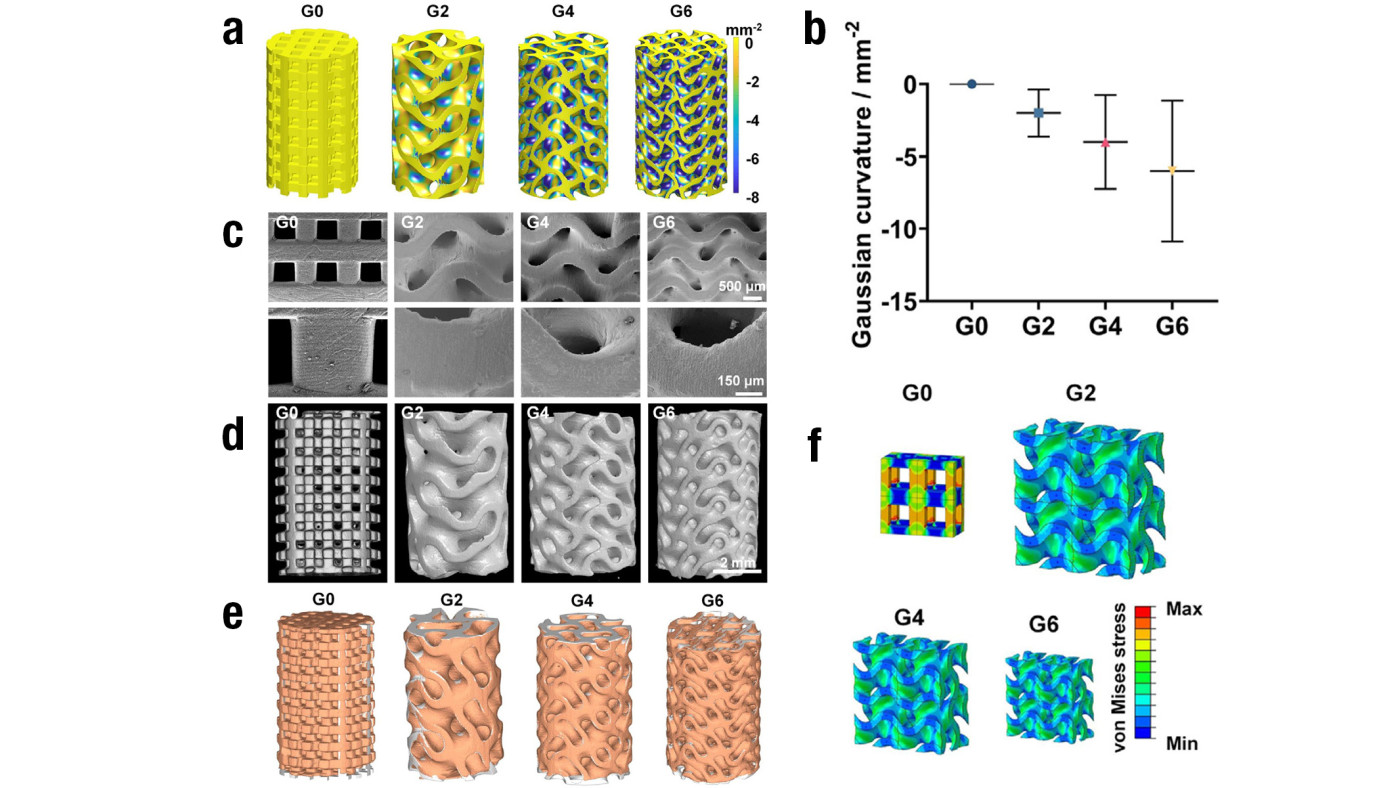
Figure 2. Characterisation of the 3D TPMS scaffolds designed by the team
a) Distribution of different Gaussian curvatures
b) Quantification of different Gaussian curvatures of the designed scaffolds
c) SEM images of the 3D-printed TPMS scaffolds of different Gaussian curvatures
d) Representative reconstructed micro-CT images of the TPMS scaffolds of different Gaussian curvatures
e) Final products of the scaffolds of different Gaussian curvatures, assessed by comparing a computer-aided design model (grey) with the corresponding 3D shapes (orange) reconstructed by micro-CT
f) von Mises stress distribution of the conventional truss scaffold (G0) and the TPMS scaffolds of different Gaussian curvatures under compression with four-unit cells analysed
The TPMS scaffolds demonstrated significantly higher compressive strength (approximately 60 MPa) compared to conventional truss scaffolds (approximately 40 MPa). This enhancement is attributed to the smoothly curved surfaces of the TPMS design, which effectively minimise stress concentration (Figure 2f). The mechanical robustness of these scaffolds is crucial for supporting the structural demands of bone tissue engineering.
In vitro studies revealed that TPMS scaffolds support the survival and proliferation of human mesenchymal stem cells (hMSCs), while substantially enhancing their osteogenic differentiation and angiogenic paracrine functions. The hyperboloidal surfaces induced distinct cellular morphologies: hMSCs exhibited a contracted shape in concave regions (negative curvature) and a snail-like configuration in convex regions (positive curvature). This behaviour is linked to curvature-induced stress fibre reorganisation, which influences cell signalling pathways.
Transcriptomic analysis identified the activation of integrin-mediated focal adhesion kinase and mitogen-activated protein kinase pathways in hMSCs cultured on TPMS scaffolds. The research team proposed that the wavy TPMS structure deforms cell nuclei into a shrunken bean-like morphology, directing cell fate towards osteogenesis and angiogenesis through integrin-mediated stress fibre reorganisation.
In vivo experiments further validated these findings. The rabbit femoral defect model demonstrated enhanced new bone formation with TPMS scaffolds, while the mouse subcutaneous implantation model confirmed their potential in supporting tissue infiltration and neovascularisation. These results underscore the scaffolds' capacity to facilitate expedited bone regeneration.
The team’s research highlights the transformative potential of TPMS scaffolds in bone tissue engineering. By mimicking hyperboloidal topography, these scaffolds induce nuclear deformation and cytoskeleton reorganisation, promoting osteogenic differentiation and angiogenic paracrine functions of hMSCs. The high porosity, improved mechanical strength, and excellent biocompatibility of TPMS scaffolds position them as a promising candidate for safer and more efficient bone grafts with significant clinical translation potential. Their findings provide a foundation for designing simple, efficient, and personalised bone grafts that simultaneously support osteogenesis and angiogenesis. The TPMS concept is also adaptable for other bone implants, including metal or polymer prostheses, broadening its applicability in regenerative medicine. As the research team continues to refine these designs, the integration of TPMS scaffolds into clinical practice could mark a significant leap forward in the treatment of bone defects and injuries.
The research is supported by the Excellent Young Scholars Projects from the National Science Foundation of China (Grant 82122002), and the Collaborative Research Fund (Grant C5044-21GF) from the Research Grants Council of Hong Kong. We also appreciate the support from the University Research Facility in 3D Printing of The Hong Kong Polytechnic University.
The data source is included in the PNAS related article and/or SI Appendix. Raw data for transcriptomic analysis were deposited under NCBI BioProject PRJNA763989 (54).
Prof. Zhao was ranked among the top 2% most-cited scientists worldwide (career-long) by Stanford University in the field of biomedical engineering in 2024, and was ranked among the top 2% most-cited scientists worldwide (single-year) for five consecutive years, from 2020 to 2024. She was named as a “Highly Cited Researcher” by Clarivate Analytics in 2022 for her significant research impact.
| References |
|---|
[1] Yang, Y., Xu, T., Bei, H., Zhang, L., Tang, C., Zhang, M., Xu, C., Bian, L., Yeung, K., Fuh, J., Zhao, X. (2022). Gaussian curvature-driven direction of cell fate toward osteogenesis with triply periodic minimal surface scaffolds. PNAS, 2022 Vol. 119 No. 41 e2206684119. https://doi.org/10.1073/pnas.2206684119
 | Prof. Xin ZHAO |



