Computationally Guided Synthesis Creates High-Efficiency Tin Halide Perovskite Nanocrystals
Other Articles

A dual-defect suppression strategy achieves record luminescence in lead-free quantum materials for advanced optoelectronics
Study conducted by Prof. Jun YINand his research team

In recent years, the world has witnessed a surge in the use of perovskite nanocrystals in optoelectronic devices that touch our daily lives, ranging from the vibrant displays of smartphones and televisions to the next generation of solar cells and photodetectors. These materials, celebrated for their exceptional light emission and tunable properties, have been at the heart of a technological revolution. However, the most efficient perovskites to date have relied heavily on lead, a toxic element that poses significant environmental and health risks. This has spurred an urgent search for safer, lead-free alternatives that do not compromise on performance.
Tin halide perovskite nanocrystals have emerged as a promising candidate in this context. Their potential to replace lead in perovskite-based devices could herald a new era of sustainable quantum materials. However, despite their promise, tin-based perovskites have been plagued by low photoluminescence quantum yields (PLQYs) and instability, limiting their practical application. The root of these challenges lies in the complex defect chemistry of tin halide perovskites, which has so far resisted conventional synthetic strategies.
To address this critical challenge, Prof. Jun YIN, Assistant Professor of the Department of Applied Physics at The Hong Kong Polytechnic University, and his research team leverage computational insights to guide the synthesis of tin halide perovskite nanocrystals with unprecedented luminescence. Their key achievement is the development of a dual-defect suppression strategy that combines tin-rich reaction conditions with the incorporation of exogenous monovalent cations. This strategy results in FASnI3 (FA = formamidinium) nanocrystals with a PLQY of 42.4% ± 1.0%, which is over 80 times higher than previously reported values for this material. This breakthrough, published in Nature Synthesis [1], not only advances the fundamental understanding of defect chemistry in tin perovskites, but also provides a practical pathway to high-performance, lead-free quantum materials for optoelectronic applications.
The aim of Prof. Yin’s study was to overcome the persistent challenge of low luminescence in tin halide perovskite nanocrystals, particularly in hybrid organic–inorganic systems. Previous attempts to enhance PLQY by adjusting precursor ratios or employing post-synthetic treatments have yielded limited success, with PLQYs rarely exceeding 20% and often remaining below 1% for hybrid systems. The team hypothesised that a synthesis strategy focused on dual-defect suppression, targeting both bulk and surface defects, could unlock the luminescent potential of tin perovskite nanocrystals. To this end, they employed density functional theory calculations to unravel the defect formation mechanisms in FASnI3 and designed a synthesis protocol that strategically combines tin-rich conditions with the introduction of monovalent cations such as 2-thiopheneethyl ammonium (TEA+) and sodium (Na+).
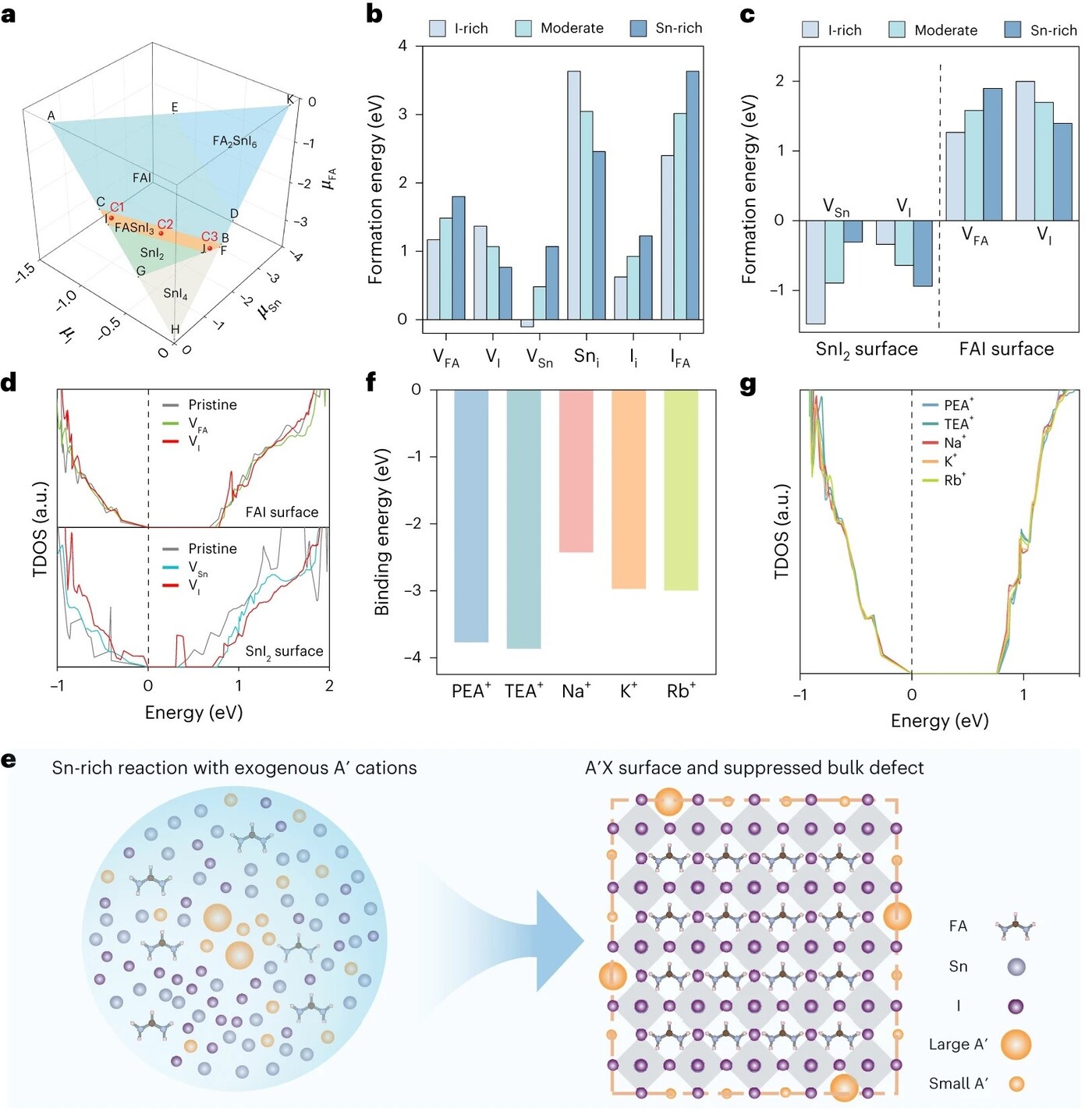
Figure 1. Computational studies and consequent synthetic strategy
a) Chemical potential region for the equilibrium growth of FASnI3 crystal
b) Formation energies for various point defects within the bulk crystal structure under conditions C1, C2, C3
c) Surface defect formation energies for VSn and VI on the Snl2 surface, and VFA and VI on the FAI surface
d) Total density of states for the supercell with the FAI surface and with the SnI2 surface in the absence and presence of surface defects
e) Schematic of the proposed synthesis strategy
f) Binding energies of PEA+, TEA+, Na+, K+ and Rb+
g) Total density of states for the supercell with the FAI surface where one FA+ is replaced by one PEA+, TEA+, Na+, K+ or Rb+
The results are both comprehensive and compelling. The computational analysis began with an exploration of the potential chemical landscape for FASnI3 growth (Figure 1a). The equilibrium region for FASnI3 formation is depicted as a slender three-dimensional polygon, within which three representative chemical potential conditions were sampled: tin-rich (C1), moderate (C2) and iodine-rich (C3). The formation energies of various bulk point defects, including antisite defects (IFA), vacancies (VI, VSn, VFA) and interstitials (Ii, Sni), were calculated under these conditions (Figure 1b). The results revealed that tin vacancies (VSn), which are particularly detrimental to photophysical properties, are most effectively suppressed under tin-rich conditions. Specifically, the formation energy of VSn reaches its lowest value (−0.1 eV) under iodine-rich conditions, but this comes at the cost of increased bulk defects elsewhere. Conversely, tin-rich conditions suppress VSn formation but do not address surface defects.
Surface defects play a critical role in non-radiative recombination in perovskite nanocrystals. The formation energies of VSn and VI at the SnI2-terminated surface, and VFA and VI at the FAI-terminated surface were evaluated under the three growth conditions (Figure 1c). The findings were striking. VSn and VI at the SnI2 surface exhibited negative formation energies across all conditions, with VI introducing deep trap states (Figure 1d), indicating poor defect tolerance. In contrast, VFA and VI at the FAI surface showed positive and substantially higher formation energies, without introducing deep traps. This suggests that an FA- and iodine-rich environment favours the formation of defect-tolerant FAI-terminated surfaces, but at the expense of increased bulk defects.
These results underscore a fundamental limitation: the chemical potentials of the constituent precursors alone cannot simultaneously suppress both bulk and surface defects. This insight led to the hypothesis that introducing exogenous monovalent cations (A′) could enable the formation of defect-tolerant A′X′ (where X′ are halides or capping ligands) surfaces under tin-rich conditions, thereby achieving dual-defect suppression (Figure 1e). The selection of suitable A′ cations was guided by computational evaluation of their binding energies and potential to introduce trap states (Figure 1f&g). TEA+ and Na⁺ emerged as optimal candidates, exhibiting favourable binding energies and no evidence of trap state formation.
The experimental realisation of this strategy involved the synthesis of FASnI3 nanocrystals using Sn(II) 2-ethylhexanoate and a SnI2-TOP (trioctylphosphine) complex as tin sources, enabling tin-rich conditions and broad tuning of reactant ratios. A′ cations were introduced via alkali metal carboxylates and organic ammonium iodides.
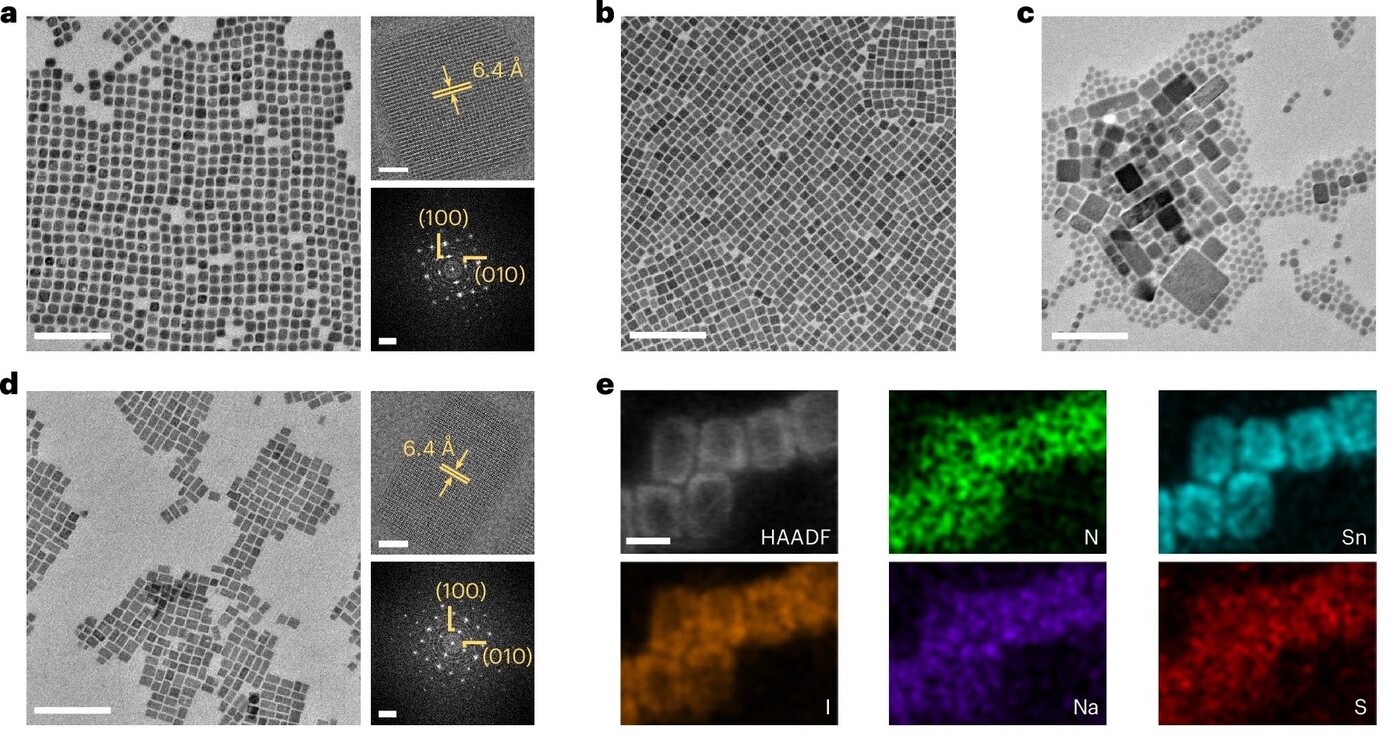
Figure 2. Morphology of as-synthesised FASnI3 nanocrystals
a) TEM image of control nanocrystals (left: scale 200 nm; upper right: scale 5 nm; lower right: scale 2nm-1)
b) TEM image of TEA nanocrystals
c) TEM image of Na nanocrystals
d) TEM image of TEA/Na nanocrystals (left: scale 200 nm; upper right: scale 5 nm; lower right: scale 2nm-1)
e) HAADF-STEM image with elemental mappings of TEA/Na nanocrystals
The control nanocrystals, synthesised without A′ cations, exhibited a single-crystalline cubic structure with a 6.4 Å interplanar spacing for the (100) plane of FASnI3 (Figure 2a). The introduction of organic cations such as TEA+ and PEA+ slightly reduced nanocrystal size without introducing two-dimensional perovskite impurities (Figure 2b). In contrast, Na+ or Rb+ yielded polydisperse nanocrystals (Figure 2c), while K+ failed to produce nanocrystals altogether. The combination of TEA+ and Na+ resulted in rod-like single-crystalline nanocrystals with the characteristic 6.4 Å spacing (Figure 2d), and elemental analysis confirmed the presence of nitrogen, tin, iodine, sodium, sulphur and phosphorus (Figure 2e).
Photophysical characterisation revealed a dramatic enhancement in PLQY upon the introduction of A′ cations. The control nanocrystals exhibited a PLQY of 0.9% ± 0.1%, with an emission peak at ~860 nm. TEA+ and PEA+ increased the PLQY to 4.0% ± 0.6% and 2.0% ± 0.2%, respectively, while Na+ alone achieved 13.6% ± 0.2%. Notably, the combination of TEA+ and Na+ yielded a PLQY of 36.9% ± 0.7%, with a further increase to 42.4% ± 1.0% upon the introduction of a small amount of chloride ions. These values represent a more than 80-fold improvement over previously reported PLQYs for FASnI3 nanocrystals. The enhanced PLQYs were corroborated by prolonged photoluminescence decay times: the average lifetime increased from 0.38 ns for the control to 132.5 ns for TEA+/Na+ nanocrystals, indicating substantially suppressed non-radiative recombination.
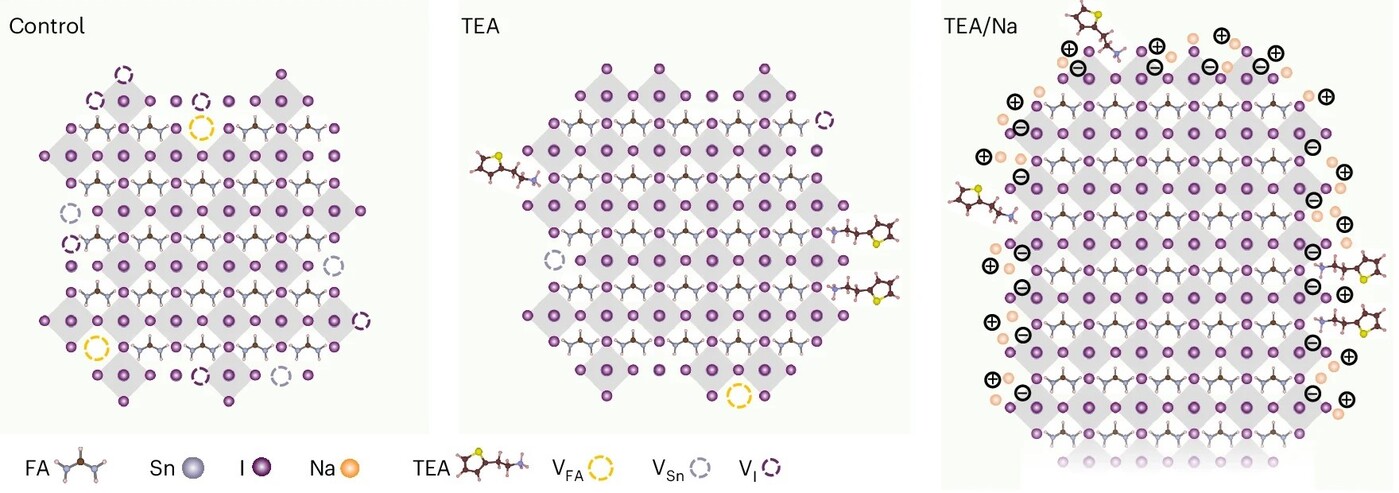
Figure 3. Proposed models for as-synthesised FASnI3 nanocrystals
The proposed structural models of the nanocrystals provide a mechanistic understanding of these enhancements (Figure 3). Under tin-rich conditions, the suppression of VSn-related bulk defects is achieved, but the resulting surfaces are deficient in FA+ and prone to defects. The inclusion of TEA+ localises some of these defects at the nanocrystal surface, promoting the formation of a TEAX′-terminated surface over the underlying SnI2 layer. However, the surface coverage of TEA+ is limited, possibly due to steric hindrance from capping ligands. The addition of the smaller Na+ ion addresses this limitation, enabling more complete surface passivation and the formation of a defect-tolerant A′X′ surface stabilised by a bipolar shell, where X′ may include iodide, oleate, 2-ethylhexanoate and/or phosphocholine. This model is supported by ζ potential measurements, which show a progression from a negatively charged, defective surface in the control (−15.0 mV) to a positively charged, defect-less surface in TEA+/Na+ nanocrystals (27.0 mV), in line with the observed increase in PLQY.
The enhancement in luminescence is further elucidated by ab initio molecular dynamics simulations, which reveal that TEA+ and Na+ adsorb onto the nanocrystal surface and enhance the integrity of the surface SnI6 octahedra. The radial distribution functions for Na–I and S–I pairs demonstrate stable binding at preferred interatomic distances, with increased Na+ concentration leading to reduced fluctuation and improved surface stabilisation. The formation energy of VI at FAI-rich surfaces increases with higher Na+ incorporation, indicating that Na+ reinforces the structural integrity of the surface. These findings suggest that the dual-cation approach not only passivates surface defects but also stabilises the nanocrystal morphology, reducing non-radiative recombination and enabling high PLQYs.
The significance of these results extends beyond the immediate system studied. The dual-defect suppression strategy is shown to be generalisable: by incorporating Cs+ under the same conditions as TEA/Na, the team synthesised phase-pure FA/Cs-alloyed tin perovskite nanocrystals, which exhibited red-shifted photoluminescence and slightly reduced PLQYs and lifetimes. The approach also enabled the synthesis of highly luminescent hybrid tin perovskite nanowires, with the underlying growth mechanism warranting further investigation. Moreover, the introduction of alternative halides further improved PLQY, with Cl0.03 nanocrystals achieving a PLQY of 42.4% ± 1.0% and a lifetime of 158.3 ns.
In conclusion, this study demonstrates that computationally guided synthesis, informed by a deep understanding of defect chemistry, can unlock the full potential of tin halide perovskite nanocrystals as high-efficiency, lead-free quantum materials.
Prof. Yin has been recognised by Stanford University as one of the top 2% most-cited scientists worldwide (career-long) in the field of nanoscience and nanotechnology in 2025, and one of the top 2% most-cited scientists worldwide (single-year) for five consecutive years, from 2021 to 2025. His research interests include perovskite materials, photophysical processes, and machine learning in materials science.
| References |
|---|
[1] Chen, JK., Zhou, Y., Zhang, BB. Kikkawa, J., Yin, J., Shirahata, N., Chen, B., Sargent, EH. & Sun, HT. Computationally guided defect-suppressing synthesis of luminescent tin halide perovskite nanocrystals. Nature Synthesis 4, 1095–1105 (2025). https://doi.org/10.1038/s44160-025-00825-4
 | Prof. Jun YIN |




