Unveiling the Real Culprit Behind Climate Change: New Insights into Black Carbon

Carbon Types Differentiation Refines Our Understanding of Climate Impacts
Study conducted by Prof. Nathanael Ling JIN and his research team

The release of carbonaceous particles into the atmosphere is a central driver of anthropogenic climate change, with black carbon (BC) recognised as one of the most potent short-lived climate forcers. Unlike greenhouse gases such as carbon dioxide, BC exerts its warming effect primarily through the absorption of sunlight, thereby heating the atmosphere and influencing cloud formation and precipitation patterns. However, the term “black carbon” encompasses a spectrum of light-absorbing carbonaceous materials, each with distinct physical and chemical properties. Among these, elemental carbon (EC) and brown carbon (BrC) are particularly significant, yet their roles in climate forcing remain incompletely understood due to the complexity of their formation and behaviour.
Traditionally, emission inventories and climate models have treated EC as a homogeneous entity, equating it with BC and assuming uniform light-absorbing properties regardless of its source or formation pathway. This simplification, while convenient, introduces substantial uncertainties into climate predictions. Recent research has highlighted that EC is not a monolithic substance but rather comprises fractions of varying maturity, reflecting the degree of graphitisation and aromatic ring condensation. Low-maturity EC, often referred to as char, and high-maturity EC, or soot, differ markedly in their optical and thermal characteristics. BrC, meanwhile, represents a class of organic carbon that absorbs light predominantly at shorter wavelengths, further complicating the picture.
The importance of distinguishing between these carbon types lies in their divergent impacts on atmospheric radiative forcing. Soot, with its highly conjugated aromatic structure, absorbs light across the visible and infrared spectrum, making it a powerful warming agent. Char and BrC, by contrast, exhibit more wavelength-dependent absorption, with BrC particularly effective in the ultraviolet and blue regions. Failing to account for these differences can lead to over- or underestimation of the climate effects of carbonaceous aerosols, especially those arising from solid fuel combustion, which is a major source of atmospheric particulate matter in many regions.
Against this backdrop, writing in Environmental, Science & Technology, a research team led by Prof. Nathanael Ling JIN, Assistant Professor of the Department of Civil and Environmental Engineering and the Department of Health Technology and Informatics at The Hong Kong Polytechnic University, seeks to unravel the formation mechanisms and optical properties of EC and BrC emitted from the combustion of solid fuels. The research aims to clarify the role of oxygenated polycyclic aromatic hydrocarbons (O-PAHs) as molecular precursors in the concurrent formation of char and BrC, and to provide empirical evidence for the need to differentiate between char and soot in climate modelling.
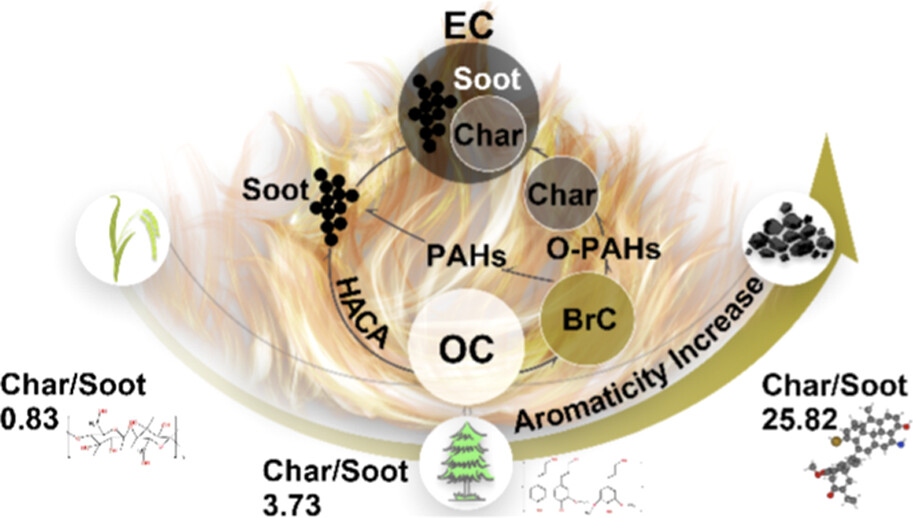
Figure 1. Three solid fuels were selected in the experiments: from the relatively low-aromaticity straw and larch wood, to the highly aromatic coal.
To probe these issues, the research team designed a series of controlled combustion experiments using three representative solid fuels: rice straw, larch wood and bituminous coal. These fuels were selected to span a broad range of chemical compositions and aromaticity, from the relatively low-aromaticity straw to the highly aromatic coal (Figure 1). This choice reflects the diversity of solid fuels commonly used in residential settings and allows for generalisable conclusions regarding the formation of carbonaceous aerosols.
Figure 2.
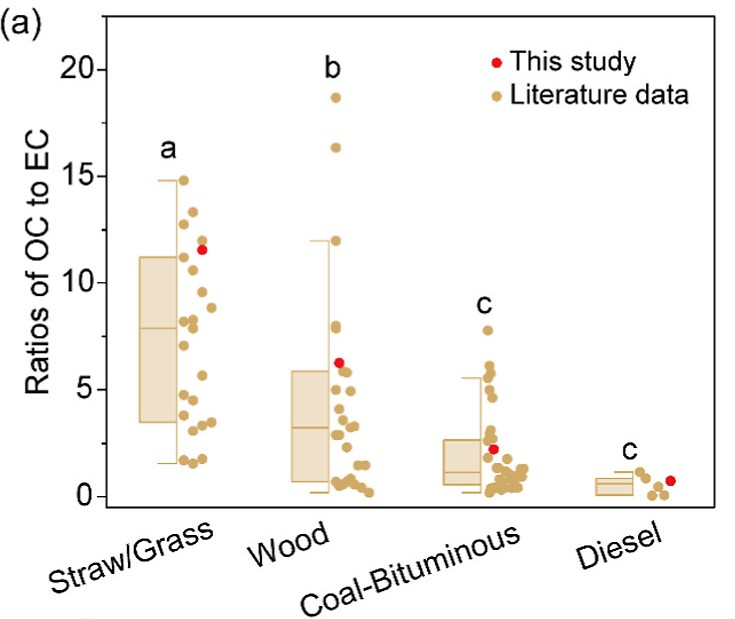
a) Comparison of ratios of OC to EC of straw, wood, coal and diesel combustion from this study and the literature.
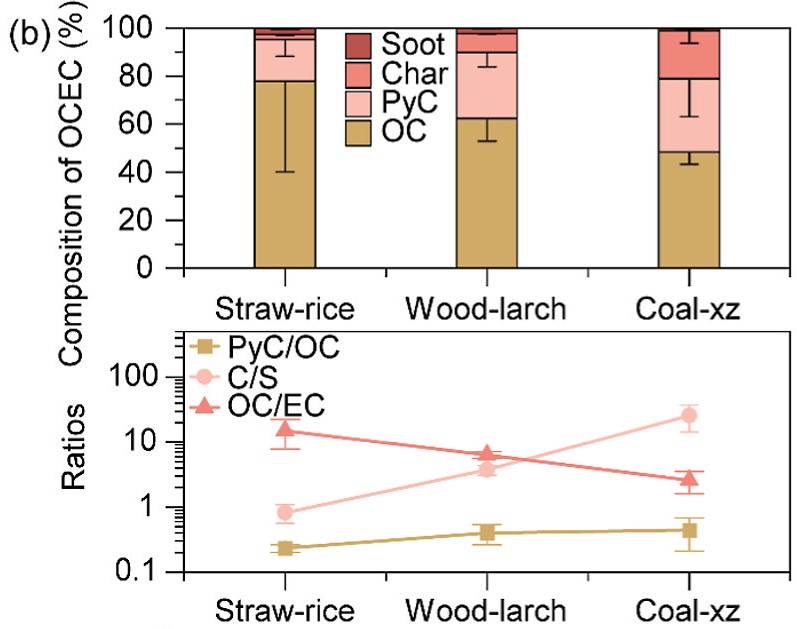
b) (above) Comparison of carbonaceous matters from the three solid fuel combustion; (below) ratios of PyC to OC, char to soot, and OC to EC from the three solid fuel combustion.
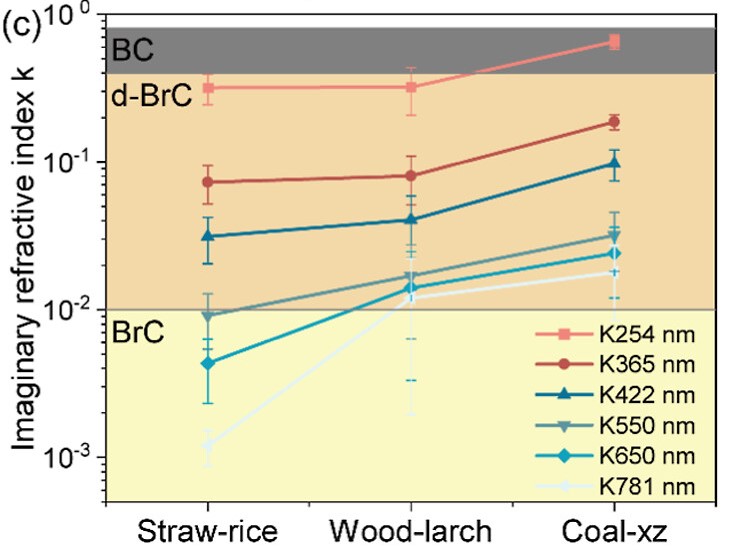
c) Imaginary part of the refract index (k) of soluble OC in particulate matter from the three solid fuel combustion at different wavelength numbers.
The research team’s results reveal a nuanced and source-dependent picture of carbonaceous aerosol formation. In the experiment, a typical high-temperature combustion source, a diesel engine, was also tested for providing a sharp contrast with solid fuel combustion. Figure 2a synthesises data from both the present study and the literature, showing that the ratio of organic carbon (OC) to elemental carbon (EC) decreases markedly from straw to wood to coal combustion, with diesel engine emissions exhibiting similarly low OC/EC ratios to those of coal. This finding challenges the conventional wisdom that high EC emissions are exclusive to high-temperature combustion sources such as diesel engines. Instead, it suggests that coal combustion, despite its lower flame temperatures, can produce substantial quantities of EC, likely due to its high aromatic content.
Exploring further, the experiment results show that the proportion of char (low-maturity EC) increases systematically with fuel aromaticity, while the soot (high-maturity EC) fraction remains relatively constant or even decreases slightly (Figure 2b). The ratio of char to soot is the highest for coal (mean value of 25.8), intermediate for wood (3.7) and lowest for straw (0.8). This trend is not simply a function of combustion temperature, as the maximum temperatures recorded for the three fuels were broadly comparable. Rather, it reflects the intrinsic chemical structure of the fuels, with more aromatic fuels promoting the formation of char through condensation and polymerisation reactions.
The optical properties of the emitted OC were assessed via the imaginary part of the refractive index (k) (Figure 2c). Here, k values at all wavelengths increase from straw to wood to coal, indicating a rise in the content of light-absorbing chromophores—molecular structures capable of absorbing visible light. Notably, the OC emitted from solid fuel combustion is more absorbing per unit carbon than standard PAHs and its absorption extends well into the visible range, suggesting the presence of highly conjugated and oxygenated aromatic structures.
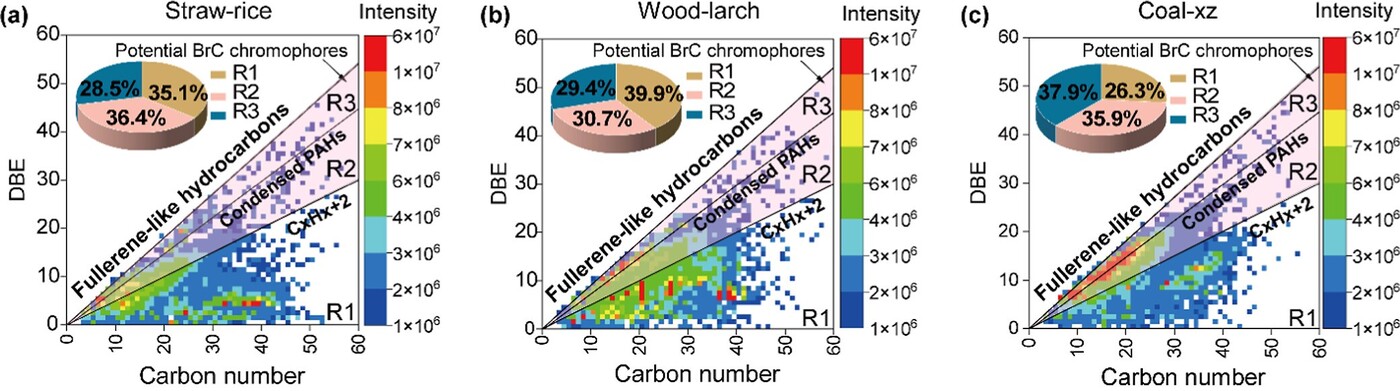
Figure 3. Double-bond equivalents (DBE) vs carbon number for assigned molecules from (a) straw-rice, (b) wood-larch, and (c) coal-xz combustion-emitted soluble organic carbon in particulate matters.
To elucidate the molecular underpinnings of these observations, FT-ICR MS was employed to map the distribution of oxygenated aromatic compounds. Figure 3 presents the double bond equivalent (DBE) versus carbon (C) number plots for the three fuels, revealing distinct chemical fingerprints. Biomass combustion produces a broad spectrum of molecules, while coal combustion is dominated by species clustering near the condensed PAH line, indicative of highly conjugated aromatic cores. The proportion of molecules with DBE/C ratios characteristic of polymerised PAHs increases from straw to coal, mirroring the trend in light absorption.
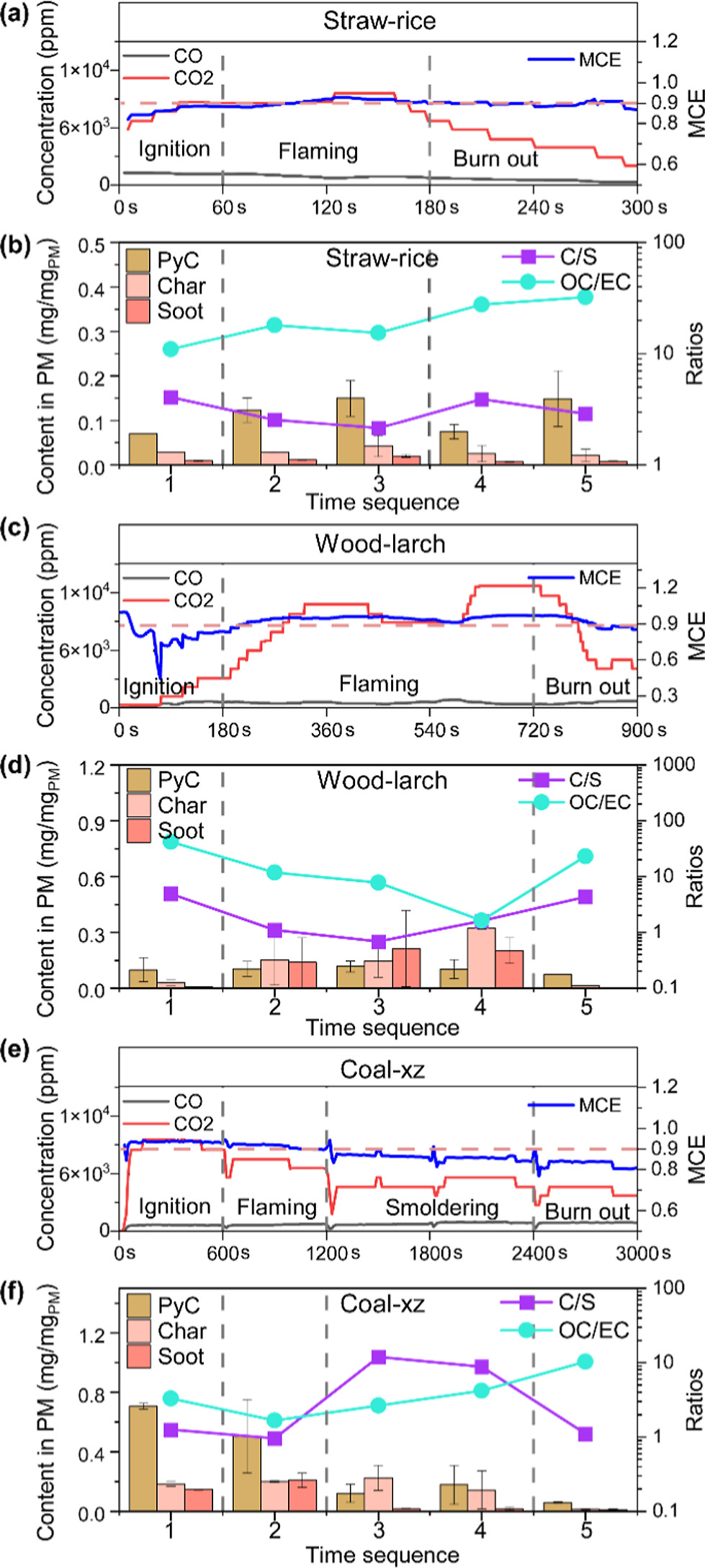
Figure 4. Variation of carbon dioxide, carbon monoxide and MCE in the flue gas (a, c, e) and time-resolved content of PyC, char, soot in particulate matters with C/S and OC/EC (b, d, f) during combustion periods of the three solid fuels.
The temporal evolution of carbonaceous emissions is captured in Figure 4, which tracks the concentrations of char, soot and their ratios across the combustion cycle. Both char and soot emissions peak during the flaming stage, corresponding to rapid decomposition and volatilisation of fuel components. However, the ratio of char to soot is inversely related to the modified combustion efficiency (MCE), with lower MCE favouring char formation. In biomass combustion, char dominates during ignition and early flaming, while in coal combustion, soot formation is initially favoured due to the abundance of volatile aromatics. However, char becomes predominant as combustion progresses and volatile content is depleted.
A key mechanistic insight emerging from the study is the central role of O-PAHs as precursors in the formation of both char and BrC. The abundance of moderately condensed O-PAHs (those with 2–10 aromatic rings) increases with fuel aromaticity and correlates strongly with both the content of pyrolysed organic carbon (PyC) and the light absorption of the extractable OC fraction. This suggests a continuum of carbonaceous species, with O-PAHs serving as intermediates in the transition from non-absorbing OC to light-absorbing BrC and ultimately to char. The presence of residual oxygen in char imparts a wavelength-dependent absorption profile, distinguishing it from the broad-spectrum absorption of soot.
In conclusion, this research underscores the importance of differentiating between char and soot. The concurrent formation of low-maturity EC (char) and BrC via O-PAH intermediates is a defining feature of solid fuel combustion, particularly for aromatic-rich fuels such as coal. By elucidating the molecular pathways and optical properties of these carbonaceous aerosols, the study provides a more refined framework for assessing their climate impacts. Incorporating these distinctions into emission inventories and radiative forcing calculations is essential for improving the accuracy of climate projections and for devising targeted mitigation strategies in regions reliant on solid fuel combustion.
Supporting information of this study is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.4c13299.
Prof. Jin is engaged in interdisciplinary research at the intersection of chemistry, toxicology and microbiology. He led a pioneering study on ecology and risks of the global plastisphere, which earned his team the Most Popular Paper Award (2020-2024) from The Innovation. Prof. Jin was named to the 2025 "40 Under 40 Recognition Program" by the American Academy of Environmental Engineers and Scientists. He is also a recipient of the Outstanding Reviewer Award from the National Science Review in 2024.
| References |
|---|
[1] Han, Y., Cai, J., Chen, Y., Zhang, Y., Jin, L. N., Chen, T., Li, J., Zhang, G., Chen, J. (2025). Concurrent Formation of Low-Maturity EC and BrC in Biomass and Coal Burning: O-PAH as a Precursor. Environmental Science & Technology May 23, 2025. https://doi.org/10.1021/acs.est.4c13299.
 | Prof. Nathanael Ling JIN
|




