Unlocking Zeolite’s Secrets: Pinpointing Aluminium Atoms in H-ZSM-5 Zeolite for a Greener Future

Revealing the Atomic Structure of Zeolites, Paving the Way for More Sustainable Industrial Catalysis
Study conducted by the late Prof. Edman Shik Chi TSANG , Prof. Benedict Tsz Woon LO
and their research team

While green energy innovations like wind turbines and electric vehicles often steal the spotlight, some of the most exciting breakthroughs in green technology are happening quietly in the world of chemistry. Crystalline materials known as zeolites, widely used as catalysts in the petrochemical industry, are now at the centre of a new wave of sustainable technology. Scientists know very little about the exact structure of zeolites. A clearer insight could make zeolites more efficient and stable catalysts. By reducing energy consumption and environmental impact, improved zeolite catalysts are paving the way for cleaner air, better fuels and a more sustainable future.
Zeolites are crystalline, sponge-like minerals with a unique ability to trap and transform molecules. They play an important role in many industrial processes. Their secret lies in their intricate, three-dimensional network of pores and channels, which can selectively host and react with different molecules. This selectivity makes zeolites indispensable as catalysts—substances that speed up chemical reactions without being consumed.
However, not all zeolites are created equal. Their catalytic power depends on the precise arrangement of atoms within their framework, especially the location of aluminium (Al) atoms. When a silicon (Si) atom in the zeolite’s silicate skeleton is replaced by an Al atom, it creates a negatively charged site that can attract protons or metal ions, forming what chemists call Brønsted acid sites (BASs). These sites are the true engines of catalysis, enabling zeolites to break and make chemical bonds with remarkable efficiency. However, the exact positions of Al atoms within the zeolite framework have long eluded scientists, limiting our ability to design better, greener catalysts.
A research team led by the late Prof. Edman Shik Chi TSANG, Chair Professor of Catalysis and Materials of the Department of Applied Biology and Chemical Technology at The Hong Kong Polytechnic University, and Prof. Benedict Tsz Woon LO, Associate Professor of the same department, has made a breakthrough in this area. The team used cutting-edge X-ray techniques to map the atomic locations of Al in a widely used zeolite called H-ZSM-5. The detailed study was published in Science [1]. This work not only deepens our understanding of zeolite chemistry but also opens up new avenues for sustainable catalysis in a low-carbon future.
To unravel the atomic structure of H-ZSM-5, the researchers turned to resonant soft X-ray diffraction (RSXRD), a powerful technique that exploits the unique way X-rays interact with specific elements. Unlike traditional X-ray diffraction, which struggles to distinguish between Al and Si atoms due to their similar scattering properties, RSXRD tunes the X-ray energy to the Al K-edge. This resonance dramatically enhances the contrast for Al atoms, allowing their positions to be pinpointed with unprecedented precision.
The team combined RSXRD with a suite of complementary methods, including neutron powder diffraction, solid-state nuclear magnetic resonance (NMR) spectroscopy and computational modelling. By integrating these methods, not only could they locate the Al atoms, but also explore how these sites interact with molecules, crucial information for understanding and improving catalytic performance.
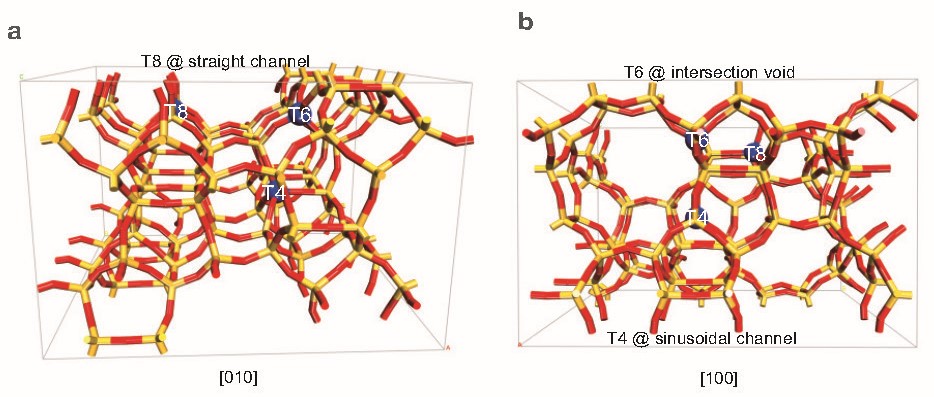
Figure 1. The refined framework Al locations at T8 (straight channel), T6 (intersection void) and T4 (sinusoidal channel) in the H-ZSM-5 zeolite viewed along the (a) [010] and (b) [100] projections
Their analysis revealed that the Al atoms in H-ZSM-5 are not randomly scattered but occupy three specific tetrahedral (T) sites within the zeolite framework: T8, T6 and T4. Each of these sites is situated in a distinct part of the zeolite’s intricate channel system. The T8 site is found in the straight channels, T6 at the intersection of straight and sinusoidal channels, and T4 deep within the sinusoidal channels (Figure 1). This precise mapping is a significant advance, as previous methods could only infer Al locations indirectly or with much less certainty.
To probe the accessibility and reactivity of these Al sites, the researchers employed two types of molecular probes: large molecules, such as trimethylphosphine oxide (TMPO), acetone and pyridine, and small molecules like ammonia, NH3. These probes act as stand-ins for reactants in real catalytic processes, revealing which sites are available for adsorption and reaction.
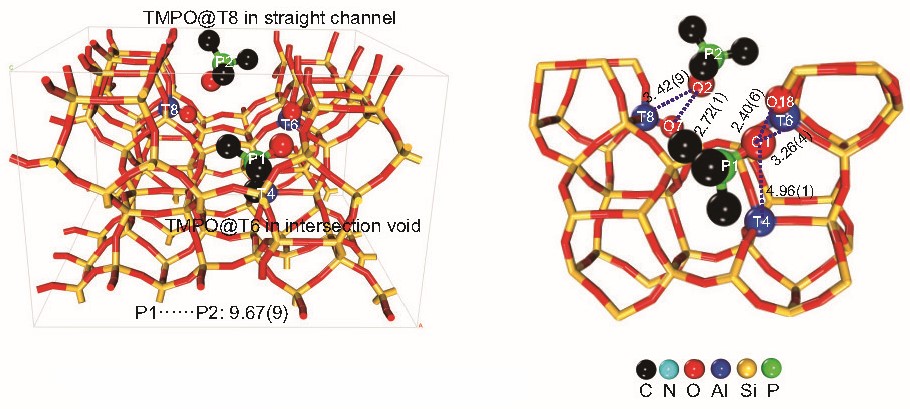
Figure 2. Indentification of acid sites on H-ZSM-5 by acetone with SXRD
The large-molecule probe studies showed that only two of the three Al sites—T8 and T6—are readily accessible to bulky molecules. When these probes were introduced in large numbers, only two were found per zeolite unit cell, despite the presence of three BAS identified by RSXRD (Figure 2). Structural analysis indicated that the T8 site, located in the straight channel, and the T6 site, at the channel intersection, could both accommodate the large probes. In contrast, the T4 site, buried in the inner sinusoidal channel, remained out of reach. This finding has important implications for catalysis: it suggests that not all acid sites in H-ZSM-5 are equally available for reactions involving larger molecules, which could influence the selectivity and efficiency of industrial processes.
To gain a more complete picture, the team turned to small-molecule probes, specifically ammonia (NH3) and its isotopic variants. Using neutron powder diffraction and advanced NMR techniques, they mapped the positions of NH3 molecules adsorbed onto the zeolite (Figure 3). Three distinct adsorption sites emerged: one where ammonia bridges between two Al atoms (T6 and T4) at the channel intersection, one where ammonia binds to a single Al atom (T8) in the straight channel and a third site where ammonia is weakly physisorbed in the intersection void.
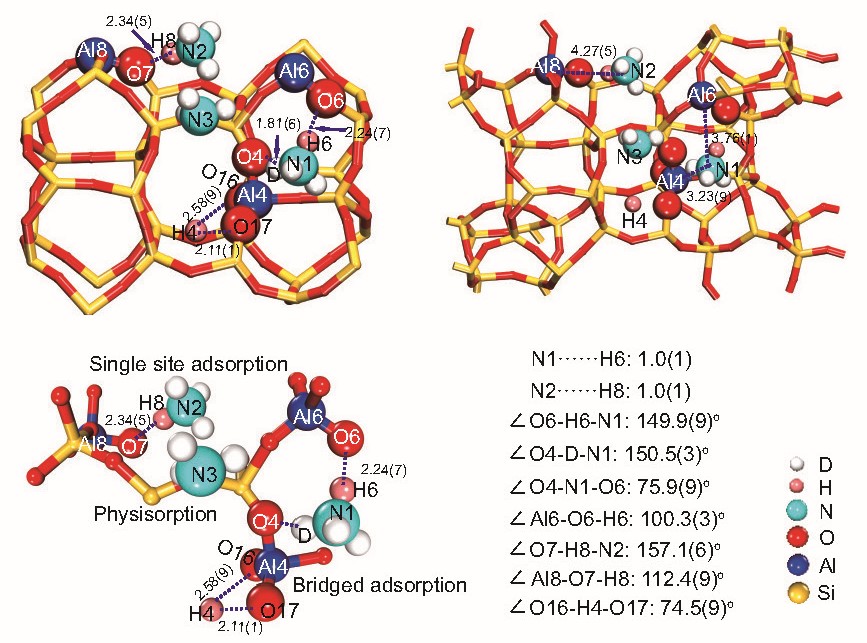
Figure 3. Refined ND3 positions in H-ZSM-5 by RSXRD and the enlarged detailed views
The most intriguing discovery was the identification of "Al pairs"—two Al atoms (at T6 and T4) in close proximity, capable of simultaneously interacting with a single NH3 molecule. This bridged adsorption mode is fundamentally different from the isolated adsorption seen at single Al sites (T8). The presence of Al pairs could enable unique catalytic pathways, particularly for reactions involving two molecules or requiring co-operative interactions. The researchers confirmed these findings with NMR experiments that measured the distances between N (from NH3) and Al atoms, providing direct evidence for the existence of Al pairs and their role in adsorption.
The study results have far-reaching implications. By precisely locating Al atoms and understanding their accessibility to different molecules, scientists can now rationally design zeolites with tailored catalytic properties. For example, by controlling the synthesis conditions to favour certain Al distributions, it may be possible to create zeolites that are optimised for specific reactions—maximising activity, selectivity and sustainability. This level of control is essential for developing greener chemical processes, reducing energy consumption and minimising waste.
In conclusion, this research marks a significant step forward in our ability to "see" and engineer the atomic landscape of zeolites. By harnessing advanced X-ray techniques and molecular probes, the research team has provided a detailed map of Al sites in H-ZSM-5 and revealed how their positions govern molecular interactions. As the world seeks cleaner, more efficient technologies, such insights will be invaluable for designing the next generation of catalysts that underpin a sustainable, low-carbon future.
The late Prof. Tsang was recognised by Stanford University as one of the top 2% most-cited scientists worldwide (career-long) in the field of organic chemistry for six consecutive years, from 2019 to 2024, and one of the top 2% most-cited scientists worldwide (single-year) for six consecutive years, from 2019 to 2024. He was also ranked among the top 10% most highly cited researchers in the general chemistry category under the Royal Society of Chemistry (RSC) and was the recipient of RSC Industry-Academia Collaboration Award, Royal Society Surfaces and Interfaces Award and Royal Society Green Chemistry Award.
Prof. Lo was the recipient of the Young Innovation Researcher Award from PolyU in 2022 and the ABCT Excellence Award 2023 (Research Output). He is also a member of RiFood at PolyU Academy for Interdisciplinary Research.
| References |
|---|
[1] Li, G., Foo, C., Fan, R., Zheng, M., Wang, Q., Chu. Y., Li, J., Day, S., Steadman, P., Tang, C., Lo, T. W. B., Deng, F., Tsang, S. C. E. (2025). Atomic locations and adsorbate interactions of Al single and pair sites in H-ZSM-5 zeolite. Science (New York, N.Y.). 387. 388-393. 10.1126/science.adq6644.
 | The Late Prof. Edman Shik Chi TSANG Department of Applied Biology and Chemical Technology |
 | Prof. Benedict Tsz Woon LO Department of Applied Biology and Chemical Technology |