Semi-Wet Carbonation: Transforming Construction Waste into Sustainable Resources

A Novel Technique Enhancing Recycled Concrete Aggregate While Capturing and Utilising CO2
Study conducted by Ir Prof. Chi-sun POONand his research team

The relentless pace of urban development has led to the proliferation of construction and demolition waste, with concrete debris accounting for approximately half of this volume. As cities expand and ageing infrastructure is dismantled, the environmental burden of concrete waste becomes increasingly pressing. Recycling concrete not only diverts substantial material from landfills but also presents a significant opportunity for reducing carbon emission.
Central to this recycling effort is the use of recycled concrete aggregate (RCA), which can serve as a substitute for natural aggregates that typically comprises 60%–80% of concrete’s volume in new construction. However, RCA’s higher porosity and water absorption compared to natural aggregates pose challenges for its widespread adoption, as these properties can compromise the mechanical strength and durability of new concrete.
To address these limitations, researchers have turned to carbonation—a process that reacts CO₂ with calcium-bearing phases in RCA, forming calcium carbonate and thereby improving the properties of an aggregate. Beyond enhancing RCA’s performance, carbonation also offers a route for permanent CO₂ sequestration, aligning with global efforts to mitigate climate change. The dual benefits of resource efficiency and carbon capture make carbonation of RCA a focus of sustainable construction research.
Traditionally, two main carbonation techniques have been explored: semi-dry carbonation and wet carbonation (Figure 1). Semi-dry carbonation employs water vapour as the reaction medium, typically under high humidity conditions (50–100% relative humidity). This method is relatively simple but suffers from slow reaction rates, with degrees of carbonation ranging from 10% to 20% after several days. The limited water availability in semi-dry carbonation restricts the diffusion of CO₂ and calcium ions, thereby impeding the formation of calcium carbonate.
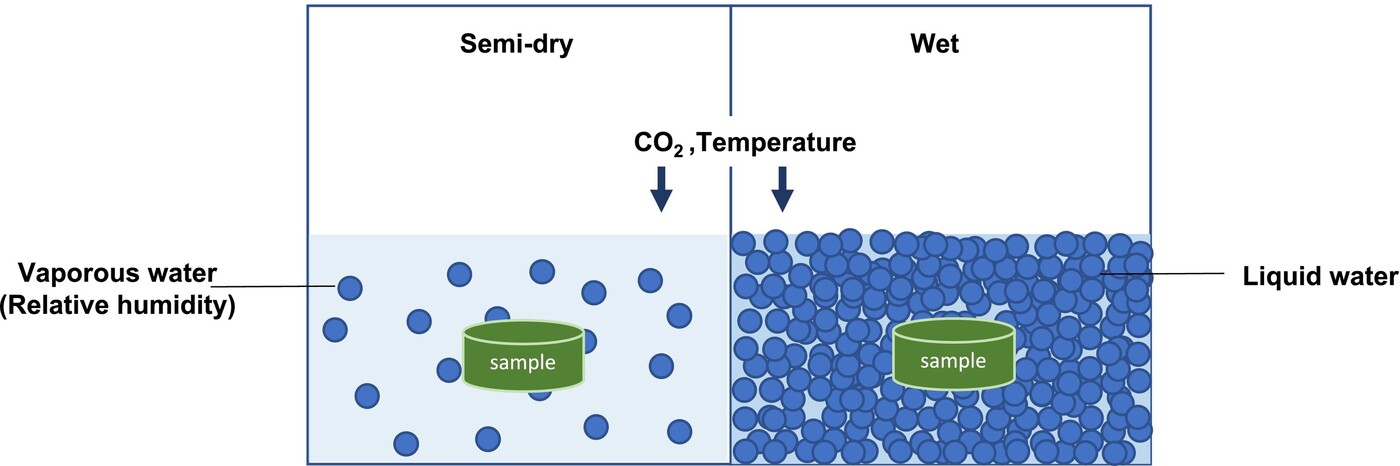
Figure 1. The medium of semi-dry carbonation process is vaporous water, while that of wet carbonation process is liquid water.
In contrast, wet carbonation immerses RCA in liquid water, facilitating faster and more complete reactions. Here, the water-to-solid ratio is a critical parameter, often ranging from 5 to 100, to ensure sufficient moisture for efficient carbonation. Wet carbonation can achieve degrees of carbonation between 10% and 20% within a few hours. However, the process is not without drawbacks: it demands significant water input and involves energy-intensive pre- and post-treatment steps such as drying, filtration and washing. These requirements complicate large-scale implementation and raise concerns about water consumption and wastewater management.
Recognising the need for a more practical and sustainable approach, Ir Prof. Chi-sun POON, Michael Anson Professor of Civil Engineering and Distinguished Research Professor of the Department of Civil and Environmental Engineering at The Hong Kong Polytechnic University, and his research team have introduced a novel semi-wet carbonation method in Cement and Concrete Research [1]. This technique bridges the gap between semi-dry and wet carbonation by employing a fine water mist at the solid-liquid interface of RCA.
In the experimental setup, RCA particles (0.3–2.36 mm) are placed in a reactor where a mist of distilled water or sodium bicarbonate solution is introduced alongside a steady flow of CO₂ at ambient temperature and pressure (Figure 2). The reactor is maintained at 100% humidity but, crucially, the water is delivered as a mist with droplet sizes of around 40 μm, significantly reducing the water-to-solid ratio compared to wet carbonation.
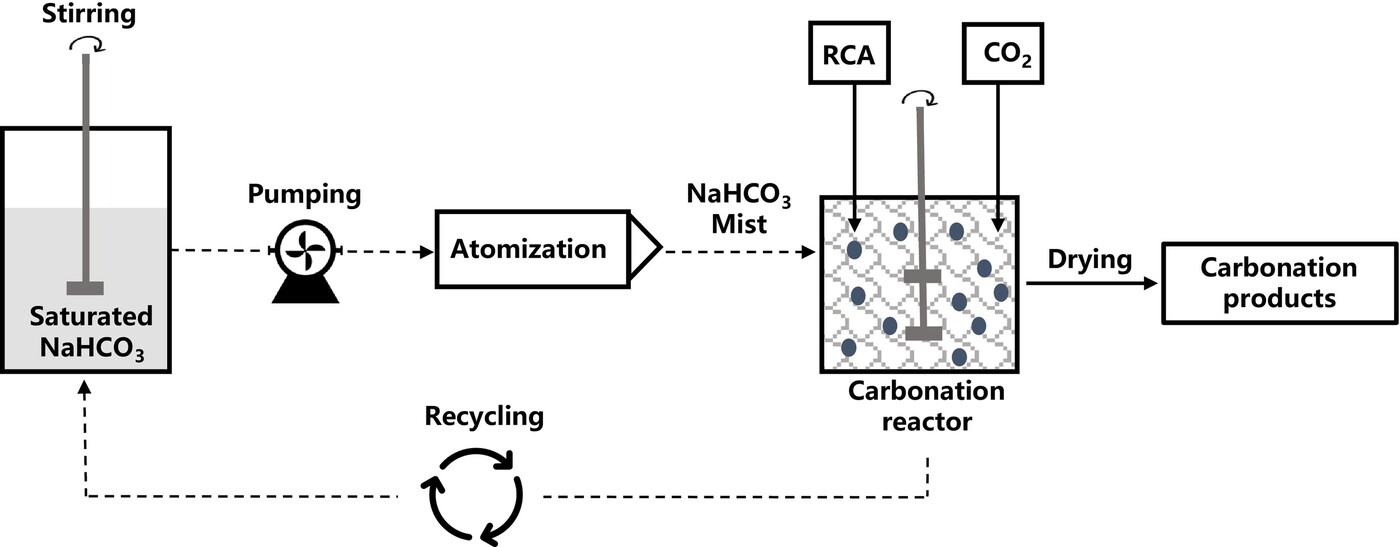
Figure 2. Workflow of semi-wet carbonation
This semi-wet environment creates a thin, spatially confined water film on the RCA surface, which proves highly effective for carbonation reactions. Remarkably, the process achieves a carbonation degree of 10.6% within just 30 minutes—comparable to, or even surpassing, the rates observed in wet carbonation under similar conditions (Figure 3).
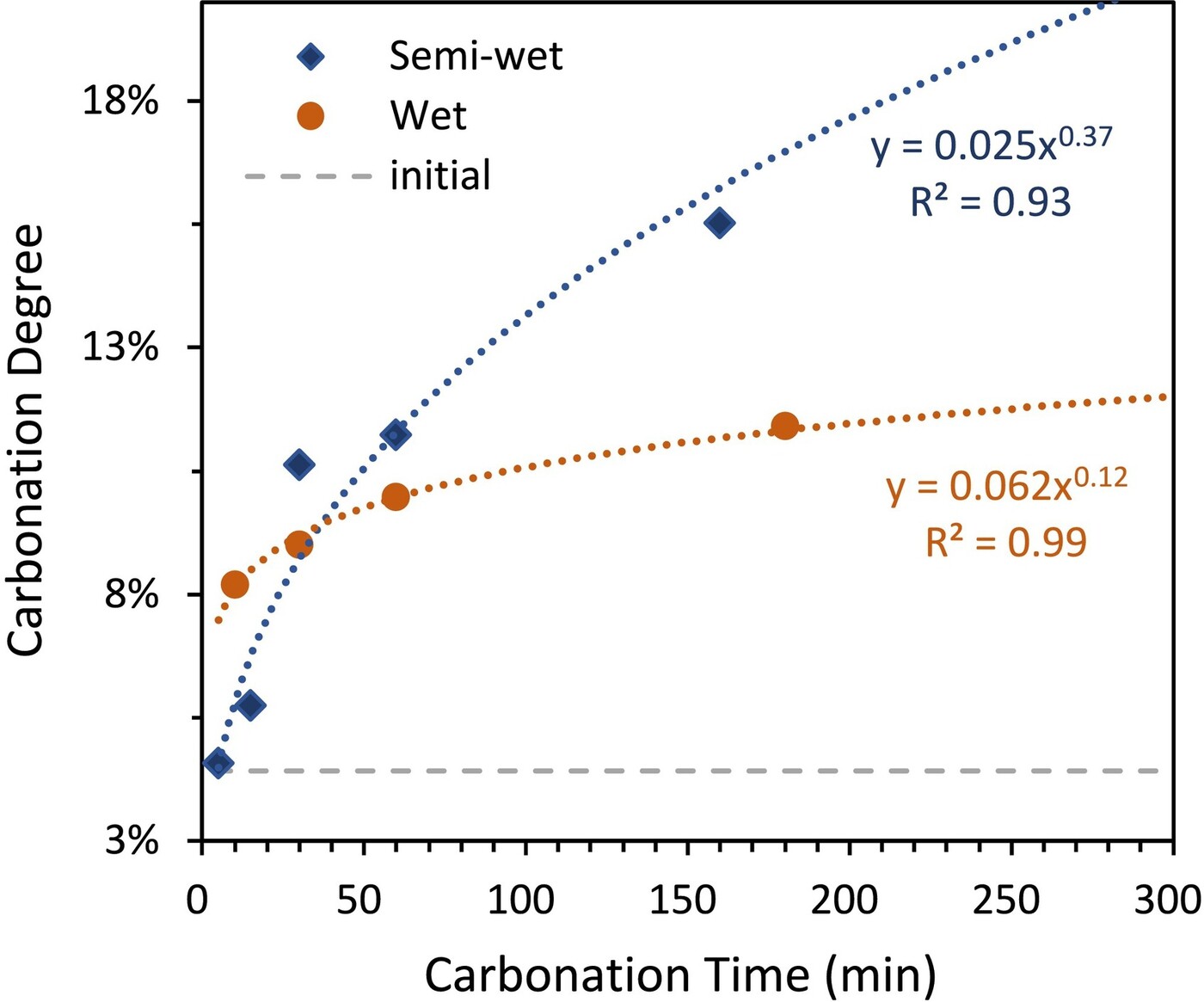
Figure 3. Performance comparison of semi-wet and wet carbonation
The semi-wet method also brings about a 3.6% reduction in water absorption and a 20% decrease in porosity, both of which are critical for improving the quality and durability of RCA in construction applications.
Another key innovation in this approach is the use of sodium bicarbonate as an accelerator. The addition of sodium bicarbonate creates a weakly alkaline environment that lowers the free energy barrier for CO₂ speciation, as confirmed by molecular dynamics simulations. This environment favours the rapid conversion of CO₂ into carbonate ions, thereby accelerating the overall carbonation process. Experimental results show that RCA treated with sodium bicarbonate mist exhibits a significantly higher increase in calcium carbonate content compared to treatment with pure water mist, underscoring the catalytic effect of the alkaline medium.
Comparative analysis between semi-wet and wet carbonation reveals several important distinctions. While both methods achieve similar degrees of carbonation in the initial stages, the semi-wet process is markedly more efficient in terms of water usage and energy consumption. The limited water in the semi-wet method not only reduces the need for post-processing but also leads to the formation of calcium carbonate with lower crystallinity and smaller grain size (0.2–1 μm), as opposed to the well-crystallised calcite produced in wet carbonation (Figure 4). This difference is attributed to the spatial confinement of the reaction zone in the semi-wet environment, which restricts crystal growth and promotes heterogeneous nucleation.

Figure 4. SEM images of RCA and calcite produced under the pure water and NaHCO3 conditions
Furthermore, the semi-wet process influences the evolution of the silicate phase in RCA. Infrared spectroscopy and solid-state nuclear magnetic resonance analyses indicate that the formation of highly polymerised silica gel is accelerated in the semi-wet environment. It suggests enhanced reactivity of the treated RCA and potentially better bonding of RCA within new concrete. Microstructural studies using a scanning electron microscope and the Brunauer-Emmett-Teller method reveal that the semi-wet method leads to a rapid reduction in pore volume—by about 20% within 30 minutes—primarily through the carbonation of mesopores and macropores. This densification further enhances the aggregate’s performance in concrete mixes.
The spatial distribution of calcium and silicon elements during carbonation also differs between the two methods. In the semi-wet process, the migration of calcium ions is limited to the immediate vicinity of the water film, resulting in localised carbonation at the solid-liquid interface. In contrast, wet carbonation allows for more extensive ion migration and deeper penetration of carbonation products into the RCA matrix.
In summary, the semi-wet carbonation technique represents a significant advancement in the sustainable utilisation of recycled concrete aggregate. By combining high carbonation efficiency with minimal water consumption and simplified processing, this method addresses the key limitations of existing carbonation strategies. The use of sodium bicarbonate as an accelerator further enhances the process, offering a practical route for industrial CO₂ capture and utilisation. As the construction industry seeks to reduce its environmental footprint, the adoption of semi-wet carbonation could play a pivotal role in converting concrete waste from a liability into a valuable resource for both material recovery and climate mitigation.
Prof. Poon has been recognised by Stanford University as one of the top 2% most-cited scientists worldwide (career-long) in the field of building and construction for six consecutive years, from 2019 to 2024, and one of the top 2% most-cited scientists worldwide (single-year) for six consecutive years, from 2019 to 2024. He was named "The Most Cited Researcher" by Elsevier in 2018 for his significant research impact. He also received an Academy Award 2017 for Best Contribution to "Sustainable Production" from the 23rd SETAC Europe LCA Case Studies Symposium and, in the same year, a Hong Kong Green Innovations Award.
| References |
|---|
[1] Gao, Y., Jiang, Y., Tao, Y., Shen, P., Poon, C.S. (2024). Accelerated carbonation of recycled concrete aggregate in semi-wet environments: A promising technique for CO2 utilization, Cement and Concrete Research, Volume 180, 2024, 107486, ISSN 0008-8846, https://doi.org/10.1016/j.cemconres.2024.107486.
 | Ir Prof. Chi-sun POON Distinguished Research Professor, |




