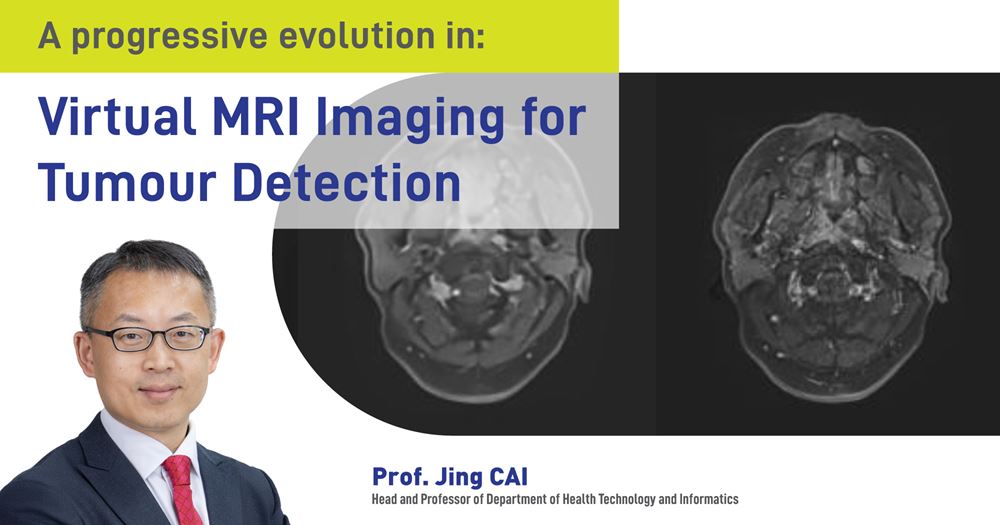
During magnetic resonance imaging (MRI) procedures, contrast agents, such as the rare metal gadolinium, can pose potential health risks. Researchers at The Hong Kong Polytechnic University (PolyU) have spent years developing contrast-free scanning technology and successfully developed AI-powered virtual MRI imaging for accurate turmour detection, offering a safer and smarter diagnostic approach.
Nasopharyngeal carcinoma (NPC) is a challenging malignancy due to its location in the nose-pharynx, a complex area surrounded by critical structures such as the skull base and cranial nerves. This cancer is particularly prevalent in Southern China, where it occurs at a rate 20 times higher than in non-endemic regions of the world, posing significant health burdens.
The infiltrative nature of NPC makes accurate imaging crucial for effective treatment planning, particularly for radiation therapy, which remains the primary treatment modality. Traditionally, contrast-enhanced MRI using gadolinium-based contrast agents (GBCAs) has been the gold standard for delineating tumour boundaries. However, the use of GBCAs carries risks, highlighting the need for safer imaging alternatives.
Gadolinium is capable of enhancing the visibility of internal structures. This is particularly useful in NPC, where the tumour's infiltrative nature requires precise imaging to distinguish it from surrounding healthy tissues. However, it also poses significant health risks, including nephrogenic systemic fibrosis. It is a serious condition associated with gadolinium exposure that leads to fibrosis of the skin, joints, and internal organs, causing severe pain and disability. Furthermore, recent studies have shown that gadolinium can accumulate in the brain, raising concerns about its long-term effects.
Prof. Jing CAI, Head and Professor of the PolyU Department of Health Technology and Informatics, has been exploring methods to eliminate the use of GBCAs, with a foucs on applying deep learning for virtual contrast enhancement (VCE) in MRI. In a paper published in International Journal of Radiation Oncology, Biology, Physics in 2022, Prof. Cai and his research team reported development of the Multimodality-Guided Synergistic Neural Network (MMgSN-Net). In 2024, he further developed the Pixelwise Gradient Model with Generative Adversarial Network (GAN) for Virtual Contrast Enhancement (PGMGVCE), as reported in Cancers.
MMgSN-Net represents a significant leap forward in synthesising virtual contrast-enhanced T1-weighted MRI images from contrast-free scans, leveraging complementary information from T1-weighted and T2-weighted images to produce high-quality synthetic images. Its architecture includes a multimodality learning module, a synergistic guidance system, a self-attention module, a multi-level module and a discriminator, all working in concert to optimise feature extraction and image synthesis. It is designed to unravel tumour-related imaging features from each input modality, overcoming the limitations of single-modality synthesis.
The synergistic guidance system plays a crucial role in fusing information from T1- and T2-weighted images, enhancing the network's ability to capture complementary features. Additionally, the self-attention module helps preserve the shape of large anatomical structures, which is particularly important for accurately delineating the complex anatomy of NPC.
Building on the foundation laid by MMgSN-Net, the PGMGVCE model introduces a novel approach to VCE in MRI imaging. This model combines pixelwise gradient methods with GAN, a deep-learning architecture, to enhance the texture and detail of synthetic images.
A GAN comprises two parts: a generator that creates synthetic images and a discriminator that evaluates their authenticity. The generator and discriminator work together, with the generator improving its outputs based on feedback from the discriminator.
In the proposed model, the pixelwise gradient method, originally used in image registration, is adept at capturing the geometric structure of tissues, while GANs ensure that the synthesised images are visually indistinguishable from real contrast-enhanced scans. The PGMGVCE model architecture is designed to integrate and prioritise features from T1- and T2-weighted images, leveraging their complementary strengths to produce high-fidelity VCE images.
In comparative studies, PGMGVCE demonstrated similar accuracy to MMgSN-Net in terms of mean absolute error (MAE), mean square error (MSE), and structural similarity index (SSIM). However, it excelled in texture representation, closely matching the texture of ground-truth contrast-enhanced images, while with MMgSN-Net the texture appears to be smoother. This was evidenced by improved metrics such as total mean square variation per mean intensity (TMSVPMI) and Tenengrad function per mean intensity (TFPMI), which indicates a more realistic texture replication. The ability of PGMGVCE to capture intricate details and textures suggests its superiority over MMgSN-Net in certain aspects, particularly in replicating the authentic texture of T1-weighted images with contrast.
Fine-tuning the PGMGVCE model involved exploring various hyperparameter settings and normalisation methods to optimise performance. The study found that a 1:1 ratio of pixelwise gradient loss to GAN loss yielded optimal results, balancing the model's ability to capture both shape and texture.
Additionally, different normalisation techniques, such as z-score, Sigmoid and Tanh, were tested to enhance the model's learning and generalisation capabilities. Sigmoid normalisation emerged as the most effective, slightly outperforming the other methods in terms of MAE and MSE.
Another aspect of the study involved evaluating the performance of the PGMGVCE model when trained with single modalities, either T1-w or T2-w images. The results indicated that using both modalities together provided a more comprehensive representation of the anatomy, leading to improved contrast enhancement when compared to using either modality alone. This finding highlights the importance of integrating multiple imaging modalities to capture the full spectrum of anatomical and pathological information.
The implications of these findings are significant for the future of MRI imaging in NPC. By eliminating reliance on GBCAs, these models offer a safer alternative for patients, particularly those with contraindications to contrast agents. Moreover, the enhanced texture representation achieved by PGMGVCE could lead to improved diagnostic accuracy, aiding clinicians in better identifying and characterising tumours.
Future research should focus on expanding these models' training datasets and incorporating additional MRI modalities to further enhance their diagnostic capabilities and generalisability across diverse clinical settings. As these technologies continue to evolve, they hold the potential to transform the medical imaging landscape, offering safer and more effective tools for cancer diagnosis and treatment planning.
Reference: Innovation Digest Issue 3
A progressive evolution in virtual MRI imaging for tumour detection
13 Aug 2025
Research and Innovation
Your browser is not the latest version. If you continue to browse our website, Some pages may not function properly.
You are recommended to upgrade to a newer version or switch to a different browser. A list of the web browsers that we support can be found here